- Completion of vaccination and enrollment in Sci-B-Vac® PROTECT and CONSTANT Phase 3 studies, respectively
- Encouraging early immunologic data observed in Phase 1/2a study of VBI-1901 for the treatment of recurrent glioblastoma
VBI Vaccines Inc. (NASDAQ: VBIV) (“VBI”), a commercial-stage biopharmaceutical company developing next-generation infectious disease and immuno-oncology vaccines, today reported financial results for the third quarter ending September 30, 2018, and provided a corporate update.
“The last few months have seen meaningful progress across our portfolio, marked by achievements in our Sci-B-Vac® Phase 3 studies, PROTECT and CONSTANT, and early data from our Phase 1/2a study of VBI-1901 in recurrent glioblastoma (GBM),” said Jeff Baxter, President and CEO. “We have now completed vaccination and enrollment in the PROTECT and CONSTANT studies, respectively, with an exceptionally low drop-out rate, and no safety signals observed or reported to-date. These accomplishments support the clean safety profile of Sci-B-Vac® and reaffirm the timeline for top-line data from the PROTECT and CONSTANT trials, expected to read out mid-year 2019 and around the 2019 year-end, respectively.”
Mr. Baxter continued, “Additionally, we are encouraged by the early immunologic data we’ve seen from our Phase 1/2a study of VBI-1901 in recurrent glioblastoma (GBM), which has shown evidence of robust boosting of CMV-specific immunity in some subjects in both the low-dose and intermediate-dose cohorts. With the addition of two top neuro-oncology sites, Dana-Farber Cancer Institute and Massachusetts General Hospital, we expect to complete enrollment in the high-dose cohort quickly. Thanks to the dedication and achievements of the entire VBI team, the fundamentals of the company remain strong and on-track as we head into a critical and exciting first half of 2019.”
Recent Highlights and Upcoming Milestones
Formation of Three Scientific and Clinical Advisory Boards
- In September, VBI announced the creation of three Scientific and Clinical Advisory Boards (SABs) comprised of leading global experts in immunology and vaccinology. The SABs will work closely with VBI’s management team to develop and advance the Company’s hepatitis B, cytomegalovirus (CMV), and glioblastoma (GBM) vaccine programs.
Appointment of Chief Financial Officer and Head of Business Development
- In August, VBI appointed Christopher McNulty as Chief Financial Officer and Head of Business Development, bringing with him a strong background in licensing transactions, capital markets and financing strategy, and public company corporate finance in the biopharmaceutical and medical device fields.
Sci-B-Vac® for Hepatitis B
- The Company’s prophylactic Hepatitis B vaccine, Sci-B-Vac®, is currently being evaluated in a global, pivotal Phase 3 clinical program, the results of which are intended to support future regulatory and marketing authorization submissions in the U.S., Europe, and Canada. The program consists of two concurrent Phase 3 studies.
- PROTECT Study: A head-to-head immunogenicity study against Engerix-B®.
- In October, VBI announced completion of vaccination, with the last subject receiving the final vaccination.
- Patients in the PROTECT study were vaccinated with a low drop-out rate of 4.5%, reinforcing the safety and tolerability of the vaccine.
- The independent Data and Safety Monitoring Board (DSMB) reviewed all PROTECT safety data available to-date and did not observe or report any safety signals.
- Top-line data from the PROTECT study are expected mid-year 2019.
- CONSTANT Study: A lot-to-lot consistency study.
- VBI completed enrollment in the CONSTANT study, with the last subject receiving the first vaccination in November.
- Top-line data from the CONSTANT study are expected around the 2019 year-end.
VBI-1901 for Glioblastoma (GBM)
- The Phase 1/2a study of VBI-1901 in recurrent GBM is ongoing, with early immunologic data to be presented in a poster discussion at the Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology (SNO) on November 16, 2018.
- Early data from the low- and intermediate-dose cohorts of VBI-1901 are encouraging, with robust boosting of CMV-specific immunity, directed against multiple antigens, observed in some subjects in both cohorts.
- In September 2018, VBI announced that the DSMB unanimously recommended continuation of the study without modification after review of all safety data from the fully-enrolled, intermediate-dose patient cohort.
- Following the DSMB recommendation, VBI initiated patient enrollment in the high-dose cohort of Part A of the study, the dose-escalation phase.
- VBI expects to complete enrollment in the high-dose study arm later in 2018, at which point a final, pre-specified DSMB review will occur before initiation of enrollment in Part B of the Phase 1/2a study.
- VBI expects to report more extensive immunologic data and 6-month overall survival and progression-free survival from all dose cohorts in Part A of the Phase 1/2a in the first half of 2019.
VBI-1501 for Congenital Cytomegalovirus (CMV)
- Following the positive safety and immunogenicity data from the Phase 1 randomized, observer-blind, placebo-controlled study evaluating the safety and immunogenicity of VBI-1501 in May 2018, discussions are ongoing with regulatory bodies to determine the design of a Phase 2 dose-ranging study.
- Further details are expected to be announced by the end of 2018.
Third Quarter 2018 Financial Results
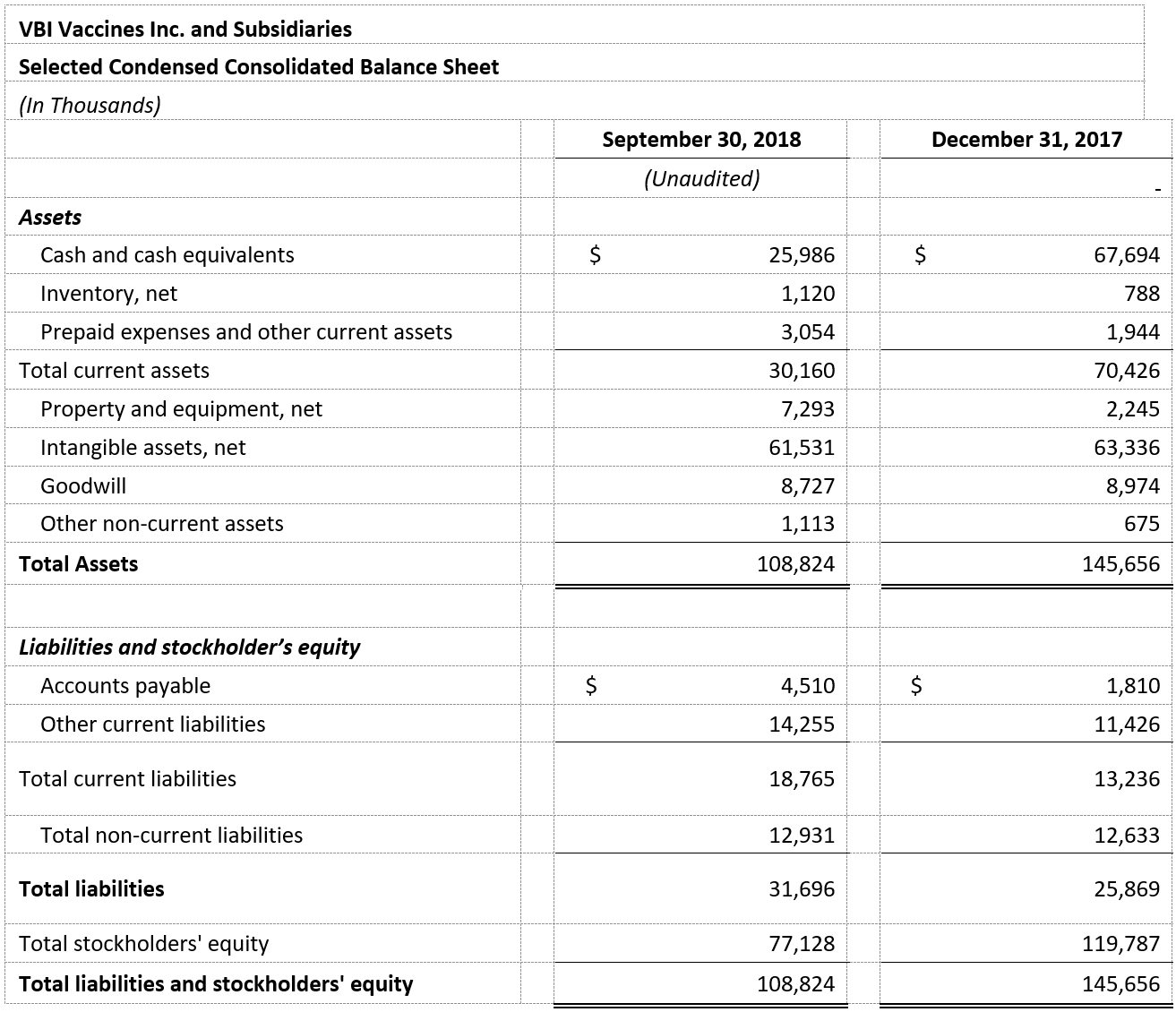
- VBI ended the third quarter of 2018 with $26.0 million in cash and cash equivalents compared with $67.7 million as of December 31, 2017. Net cash used in operations for the nine months ended September 30, 2018 was $38.0 million.
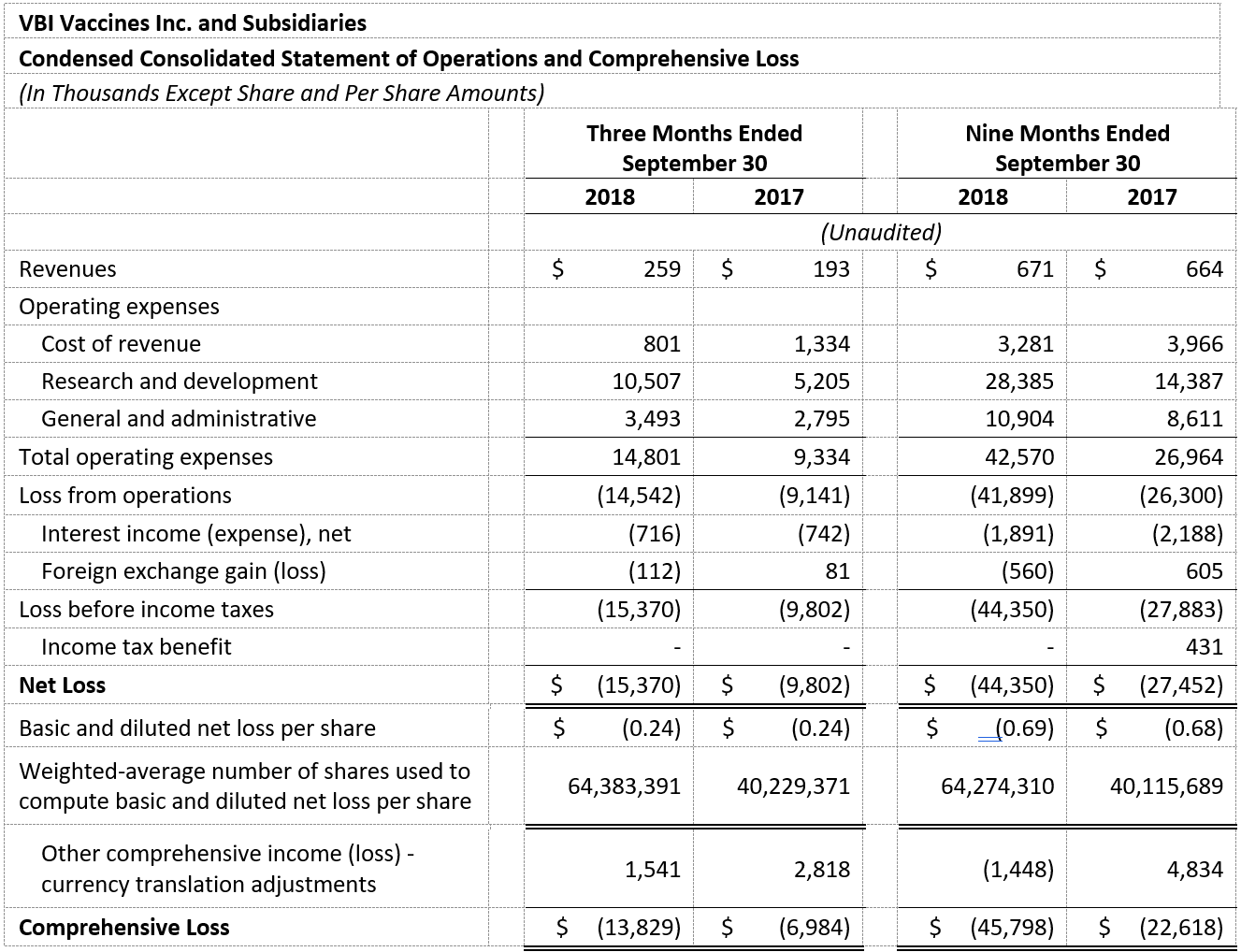
- Revenue for the third quarter of 2018 was $0.26 million and was primarily attributable to sales of Sci-B-Vac in Israel and in Europe on a named-patient basis. Revenue for the third quarter of 2017 was $0.19 million and was primarily attributable to sales of Sci-B-Vac in Israel.
- Research and development expenses were $10.5 million for the third quarter of 2018, as compared to $5.2 million for the same period in 2017. The increase was primarily due to the continuation of the Phase 3 program for Sci-B-Vac® and the Phase 1/2a clinical study for VBI-1901 in recurrent GBM patients.
- General and administrative expenses were $3.5 million for the third quarter of 2018 as compared to $2.8 million for the same period in 2017. The increase was primarily due to allocation of expenses from costs of revenues related to the modernization and capacity increases of our manufacturing facility in Rehovot, Israel, human resources expenses, and stock-based compensation expenses.
- Net loss and net loss per share for the third quarter of 2018 were $15.4 million and $0.24 respectively, compared to a net loss of $9.8 million and a net loss per share of $0.24 for the third quarter of 2017.
About VBI Vaccines Inc.
VBI Vaccines Inc. (“VBI”) is a commercial-stage biopharmaceutical company developing a next generation of vaccines to address unmet needs in infectious disease and immuno-oncology. VBI’s first marketed product is Sci-B-Vac®, a hepatitis B (HBV) vaccine that mimics all three viral surface antigens of the hepatitis B virus; Sci-B-Vac® is approved for use in Israel and 10 other countries. VBI’s eVLP Platform technology enables the development of enveloped virus-like particle (eVLP) vaccines that closely mimic the target virus to elicit a potent immune response. VBI is advancing a pipeline of eVLP vaccines, with lead programs in cytomegalovirus (CMV) and glioblastoma (GBM). VBI is headquartered in Cambridge, MA with research operations in Ottawa, Canada and research and manufacturing facilities in Rehovot, Israel.
Cautionary Statement on Forward-looking Information
Certain statements in this press release that are forward-looking and not statements of historical fact are forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are forward-looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). The company cautions that such statements involve risks and uncertainties that may materially affect the company’s results of operations. Such forward-looking statements are based on the beliefs of management as well as assumptions made by and information currently available to management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, including but not limited to the ability to establish that potential products are efficacious or safe in preclinical or clinical trials; the ability to establish or maintain collaborations on the development of therapeutic candidates; the ability to obtain appropriate or necessary governmental approvals to market potential products; the ability to obtain future funding for developmental products and working capital and to obtain such funding on commercially reasonable terms; the company’s ability to manufacture product candidates on a commercial scale or in collaborations with third parties; changes in the size and nature of competitors; the ability to retain key executives and scientists; and the ability to secure and enforce legal rights related to the company’s products. A discussion of these and other factors, including risks and uncertainties with respect to the company, is set forth in the Company’s filings with the Securities and Exchange Commission and the Canadian securities authorities, including its Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 26, 2018, and filed with the Canadian security authorities at sedar.com on February 26, 2018, as may be supplemented or amended by the Company’s Quarterly Reports on Form 10-Q. Given these risks, uncertainties and factors, you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. All such forward-looking statements made herein are based on our current expectations and we undertake no duty or obligation to update or revise any forward-looking statements for any reason, except as required by law.
VBI Contact
Nicole Anderson, Communications Executive
Phone: (617) 830-3031 x124
Email: info@vbivaccines.com
VBI Investor Contact
Nell Beattie
Chief Business Officer
Email: IR@vbivaccines.com
VBI Media Contact
Burns McClellan, Inc.
Robert Flamm, Ph.D.
Phone: (212) 213-0006
Email: rflamm@burnsmc.com









