- Pivotal Phase 3 program for Sci-B-Vac® Hepatitis B vaccine continues to advance towards topline data expected mid-year 2019
- Positive safety and immunogenicity data from Phase 1 study of VBI-1501 for Congenital Cytomegalovirus (CMV) reported in May 2018
- Initial data from Phase 1/2a study of VBI-1901 for the treatment of recurrent glioblastoma (GBM) expected H2 2018
VBI Vaccines Inc. (NASDAQ: VBIV) (“VBI”), a commercial-stage biopharmaceutical company developing next-generation infectious disease and immuno-oncology vaccines, today reported financial results for the second quarter ending June 30, 2018, and provided a corporate update.
“We continue to make strong progress in all three of our lead clinical programs,” said Jeff Baxter, President and CEO of VBI. “Our pivotal Phase 3 program of Sci-B-Vac®, our hepatitis B vaccine, is advancing well towards topline data from the PROTECT study expected mid-year 2019. Our eVLP technology-based pipeline showed exciting results in the clinic in May, when we reported positive final safety and immunogenicity data from our Phase 1 randomized study of VBI-1501 for CMV. This study was the first in-human study of a vaccine developed with our eVLP technology. We were excited to be able to demonstrate a robust immunologic response at a dose that is one-tenth the amount of other organizations’ CMV vaccine candidates. For our immuno-oncology pipeline, also developed with our eVLP technology, we look forward to reporting initial immunologic data from the Phase 1/2a study of VBI-1901 in recurrent GBM patients, which we expect in the second half of this year.”
Recent Highlights and Upcoming Milestones
Sci-B-Vac® for Hepatitis B
- Sci-B-Vac®, the Company’s Hepatitis B vaccine, is currently being studied in a pivotal Phase 3 clinical program in the U.S., Europe, and Canada. This program consists of two concurrent Phase 3 studies, the PROTECT study and the CONSTANT study.
- Topline data from the PROTECT study are expected mid-year 2019, with topline data from the CONSTANT study expected in the second half of 2019.
- The results from this pivotal Phase 3 program are intended to support future regulatory filings in the U.S., Europe, and Canada.
VBI-1501 for Congenital Cytomegalovirus (CMV)
- In May 2018, VBI reported positive safety and immunogenicity data from a Phase 1 randomized, observer-blind, placebo-controlled study designed to evaluate the safety, tolerability, and immunogenicity of VBI-1501 in 128 CMV-negative, healthy adults aged 18-40 years.
- Subjects were randomized into five arms to receive various dose levels of a modified form of the CMV gB antigen, gB-g, with or without the adjuvant alum, or to receive placebo. The highest does of antigen tested was 2.0μg of gB-g content with alum (VBI-1501A 2.0μg).
- The final Phase 1 study results demonstrated that VBI-1501A was safe and well-tolerated at all doses.
- VBI-1501A 2.0μg elicited CMV-neutralizing antibodies against fibroblast cell infection in 100% of subjects after the third vaccination (85% after two vaccinations), inducing titers comparable to those observed in patients protected as a result of natural infection (CMV-positive controls).
- In epithelial cells, which have historically been the much harder cell type to protect against infection, VBI-1501A 2.0μg demonstrated a 31% neutralizing antibody seroconversion rate after three vaccinations, up from 17% after two vaccinations.
- Discussions with regulatory bodies are planned for the second half of 2018 to discuss the next stage of development for VBI-1501A.
VBI-1901 for Glioblastoma (GBM)
- A Phase 1/2a Clinical Study of VBI-1901 for the treatment of recurrent glioblastoma (rGBM) is ongoing.
- The multi-center, open-label, two-part study will enroll up to 28 patients and is designed to evaluate safety, tolerability, and the optimal therapeutic dose level of VBI-1901.
- Extensive biomarker testing and tumor imaging is built into this clinical protocol to enable the Company to assess the robustness of the immune response induced by VBI-1901.
- In the second half of 2018, VBI hopes to be able to correlate this immunologic and biomarker data with initial clinical outcomes from the low and mid dose cohorts, and expects 6-month overall survival (OS) and progression-free survival (PFS) data in the first half of 2019.
First Quarter 2018 Financial Results
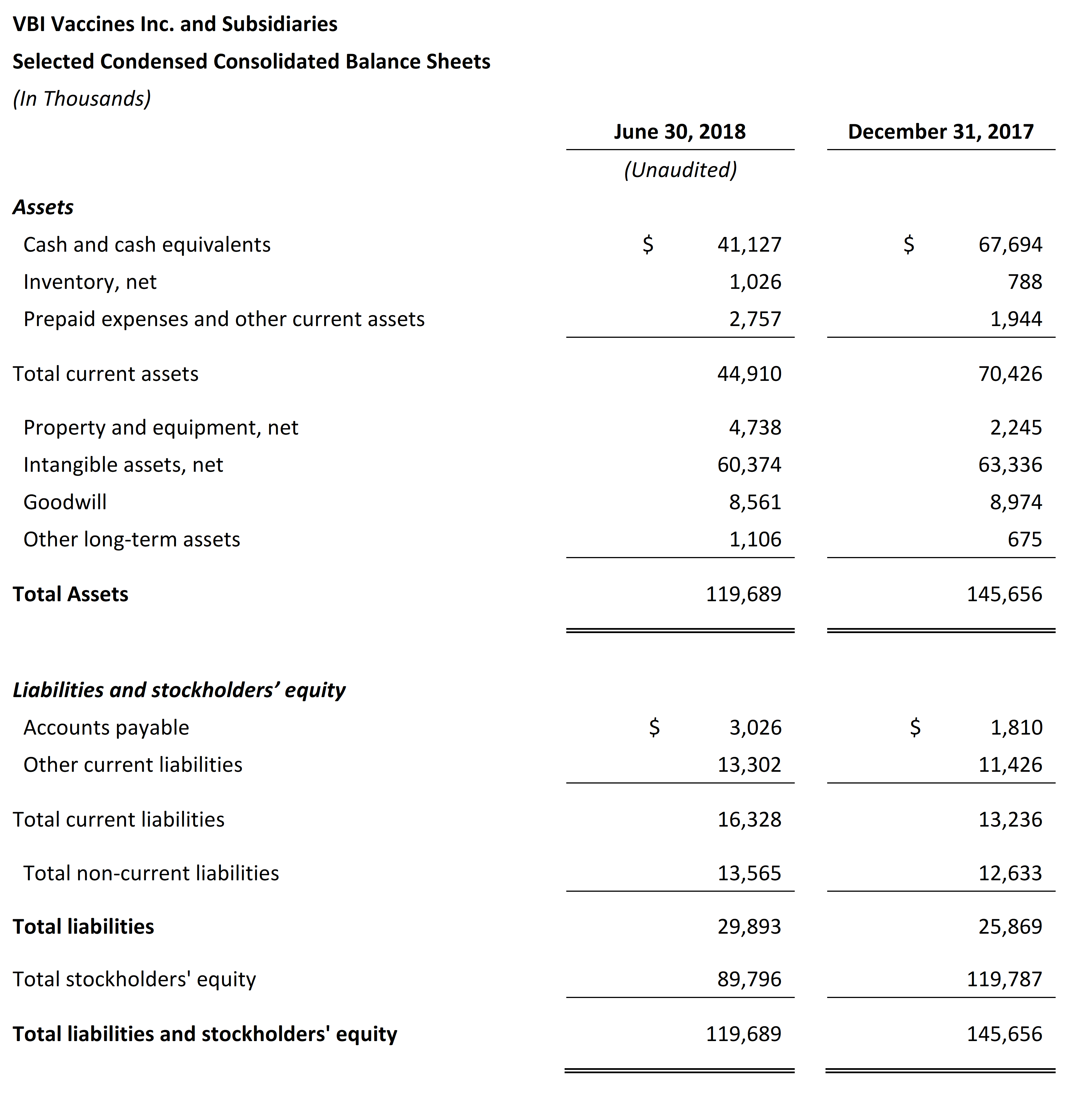
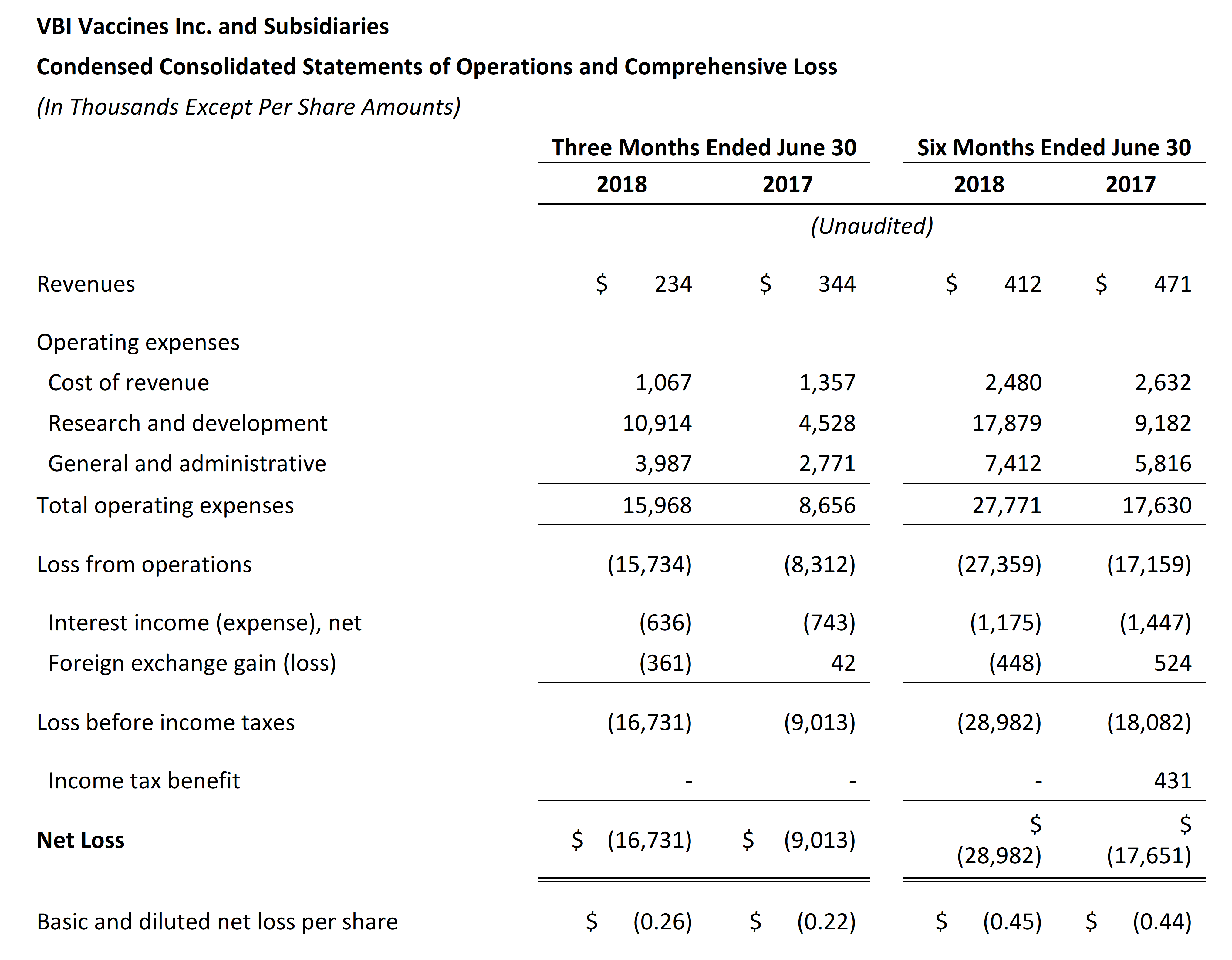
- VBI ended the second quarter of 2018 with $41.1 million in cash and cash equivalents compared with $67.7 million as of December 31, 2017. Net cash used in operations for the six months ended June 30, 2018 was $24.5 million.
- Revenue for the second quarter of 2018 was $0.2 million and was primarily attributable to sales of Sci-B-Vac in Israel and in Europe, on a named-patient basis. Revenue for the second quarter of 2017 was $0.3 million and was primarily attributable to sales of Sci-B-Vac in Israel and to a services agreement that was completed during that time frame.
- Research and development expenses were $10.9 million for the second quarter of 2018, as compared to $4.5 million for the same period in 2017. The increase was primarily due to the continuation of the Phase 3 program for Sci-B-Vac®and the Phase 1/2a clinical study for VBI-1901 in recurrent GBM patients, but was partially offset by the reduction in research expenses due to the advancement of VBI-1901 into clinical development from pre-clinical development.
- General and administrative expenses were $4.0 million for the second quarter of 2018, as compared to $2.8 million for the same period in 2017. The increase was primarily due to human resources expenses, stock-based compensation expenses, and impairment related to leasehold improvements and manufacturing equipment no longer being utilized in the business as a result of the renovation and capacity increases of our manufacturing facility in Rehovot, Israel.
- Net loss and net loss per share for the second quarter of 2018 were $16.7 million and $0.26, respectively, compared to a net loss of $9.0 million and a net loss per share of $0.22 for the second quarter of 2017.
About VBI Vaccines Inc.
VBI Vaccines Inc. (“VBI”) is a commercial-stage biopharmaceutical company developing a next generation of vaccines to address unmet needs in infectious disease and immuno-oncology. VBI’s first marketed product is Sci-B-Vac®, a hepatitis B (HBV) vaccine that mimics all three viral surface antigens of the hepatitis B virus; Sci-B-Vac® is approved for use in Israel and 10 other countries. VBI’s eVLP Platform technology enables the development of enveloped virus-like particle (eVLP) vaccines that closely mimic the target virus to elicit a potent immune response. VBI is advancing a pipeline of eVLP vaccines, with lead programs in cytomegalovirus (CMV) and glioblastoma (GBM). VBI is headquartered in Cambridge, MA with research operations in Ottawa, Canada and research and manufacturing facilities in Rehovot, Israel.
Cautionary Statement on Forward-looking Information
Certain statements in this press release that are forward-looking and not statements of historical fact are forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are forward-looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). The company cautions that such statements involve risks and uncertainties that may materially affect the company’s results of operations. Such forward-looking statements are based on the beliefs of management as well as assumptions made by and information currently available to management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, including but not limited to the ability to establish that potential products are efficacious or safe in preclinical or clinical trials; the ability to establish or maintain collaborations on the development of therapeutic candidates; the ability to obtain appropriate or necessary governmental approvals to market potential products; the ability to obtain future funding for developmental products and working capital and to obtain such funding on commercially reasonable terms; the company’s ability to manufacture product candidates on a commercial scale or in collaborations with third parties; changes in the size and nature of competitors; the ability to retain key executives and scientists; and the ability to secure and enforce legal rights related to the company’s products. A discussion of these and other factors, including risks and uncertainties with respect to the company, is set forth in the Company’s filings with the Securities and Exchange Commission and the Canadian securities authorities, including its Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 26, 2018, and filed with the Canadian security authorities at sedar.com on February 26, 2018, as may be supplemented or amended by the Company’s Quarterly Reports on Form 10-Q. Given these risks, uncertainties and factors, you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. All such forward-looking statements made herein are based on our current expectations and we undertake no duty or obligation to update or revise any forward-looking statements for any reason, except as required by law.
VBI Contact
Nicole Anderson, Communications Executive
Phone: (617) 830-3031 x124
Email: info@vbivaccines.com
VBI Investor Contact
Nell Beattie
Chief Business Officer
Email: IR@vbivaccines.com
VBI Media Contact
Burns McClellan, Inc.
Robert Flamm, Ph.D.
Phone: (212) 213-0006
Email: rflamm@burnsmc.com









