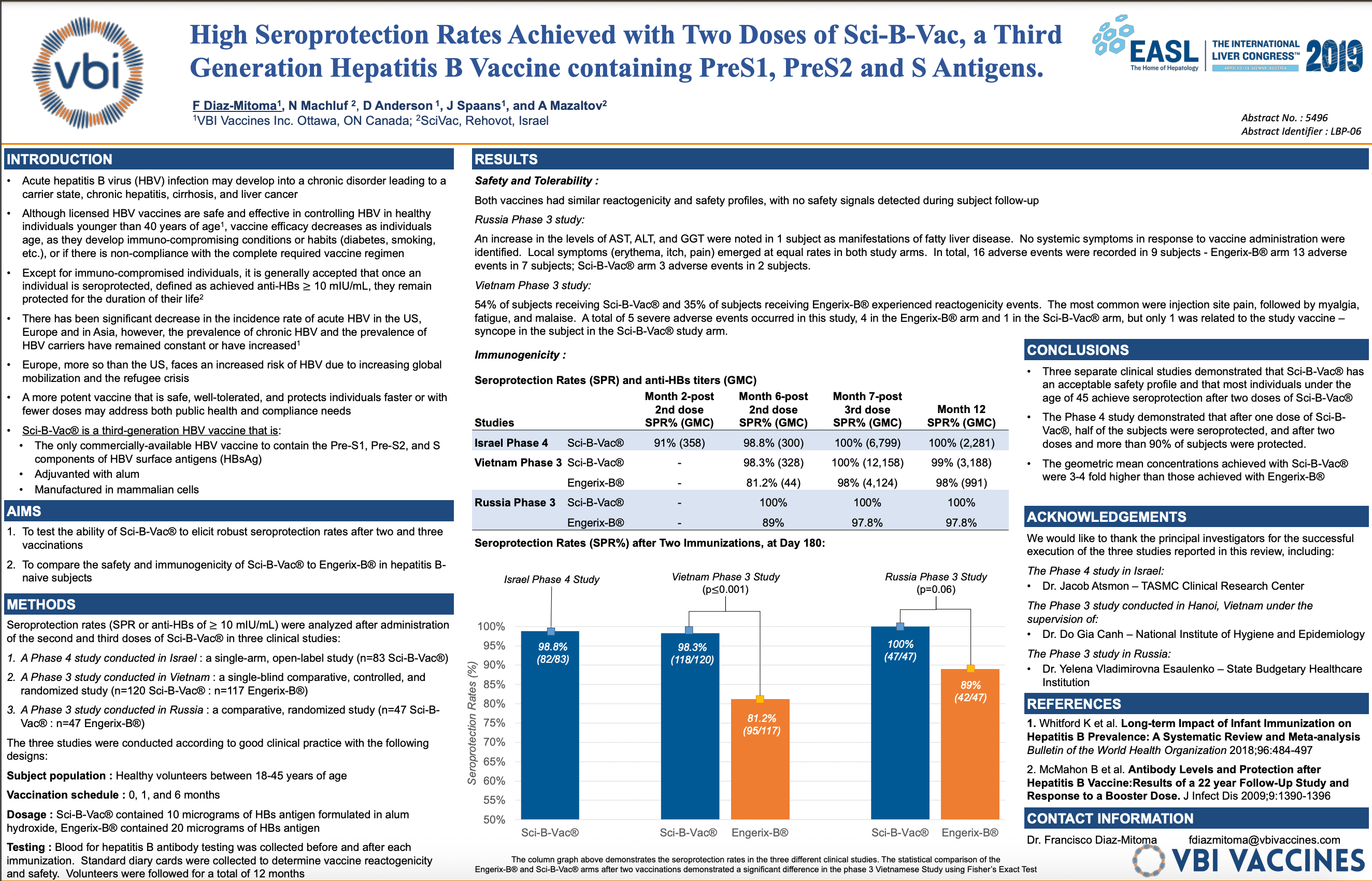
- Poster presentation to highlight data from three previously-conducted clinical studies of Sci-B-Vac®, VBI’s prophylactic Hepatitis B vaccine
- In all three studies, seroprotection rates of more than 98% were achieved after two vaccinations of Sci-B-Vac®
VBI Vaccines Inc. (NASDAQ: VBIV) (“VBI”) (“the Company”), a commercial-stage biopharmaceutical company developing next-generation infectious disease and immuno-oncology vaccines, today announced that the Company’s abstract related to the prophylactic hepatitis B vaccine, Sci-B-Vac®, has been selected for a late-breaking poster presentation at The International Liver Congress™ (ILC), the Annual Meeting of the European Association for the Study of the Liver (EASL) to be held in Vienna, Austria, April 10-14, 2019.
Dr. Francisco Diaz-Mitoma, M.D., Ph.D., VBI’s Chief Medical Officer, will present the poster which highlights safety and immunogenicity data, in subjects aged 18 to 45 years after receiving two and three doses of Sci-B-Vac®, from three previously-conducted clinical studies – two randomized Phase 3 studies comparing Sci-B-Vac® to Engerix-B® conducted in Vietnam and Russia, and one single-arm Phase 4 study conducted in Israel. All three studies demonstrated a clean safety profile for Sci-B-Vac® and seroprotection rates of more than 98% after two vaccinations in all subjects receiving Sci-B-Vac®.
Poster Presentation Details
- LBP-06
- Title: High Seroprotection Rates Achieved with Two Doses of Sci-B-Vac, a Third Generation Hepatitis B Vaccine Containing PreS1, PreS2, and S Antigens
- Session: Late-breaker poster
- Date: Thursday, April 11, 2019 – Saturday, April 13, 2019
- Time: 9:00 AM to 5:00 PM CEST
About VBI Vaccines Inc.
VBI Vaccines Inc. (Nasdaq: VBIV) is a commercial-stage biopharmaceutical company developing a next generation of vaccines to address unmet needs in infectious disease and immuno-oncology. VBI is advancing the prevention and treatment of hepatitis B, with the only commercially-approved trivalent hepatitis B vaccine, Sci-B-Vac®, which is approved for use in Israel and 10 other countries and is currently in a Phase 3 study in the U.S., Europe, and Canada, and with an immuno-therapeutic in development for a functional cure for chronic hepatitis B. VBI’s eVLP Platform technology allows for the development of enveloped virus-like particle (eVLP) vaccines that closely mimic the target virus to elicit a potent immune response. Integrating its cytomegalovirus (CMV) expertise with the eVLP platform technology, VBI’s lead eVLP vaccine candidates include a prophylactic CMV vaccine candidate and a therapeutic glioblastoma (GBM) vaccine candidate. VBI is headquartered in Cambridge, MA with research operations in Ottawa, Canada and research and manufacturing facilities in Rehovot, Israel.
Cautionary Statement on Forward-looking Information
Certain statements in this press release that are forward-looking and not statements of historical fact are forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are forward-looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). The company cautions that such statements involve risks and uncertainties that may materially affect the company’s results of operations. Such forward-looking statements are based on the beliefs of management as well as assumptions made by and information currently available to management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, including but not limited to the ability to establish that potential products are efficacious or safe in preclinical or clinical trials; the ability to establish or maintain collaborations on the development of therapeutic candidates; the ability to obtain appropriate or necessary governmental approvals to market potential products; the ability to obtain future funding for developmental products and working capital and to obtain such funding on commercially reasonable terms; the company’s ability to manufacture product candidates on a commercial scale or in collaborations with third parties; changes in the size and nature of competitors; the ability to retain key executives and scientists; and the ability to secure and enforce legal rights related to the company’s products. A discussion of these and other factors, including risks and uncertainties with respect to the company, is set forth in the Company’s filings with the Securities and Exchange Commission and the Canadian securities authorities, including its Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 25, 2019, and filed with the Canadian security authorities at sedar.com on February 25, 2019, as may be supplemented or amended by the Company’s Quarterly Reports on Form 10-Q. Given these risks, uncertainties and factors, you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. All such forward-looking statements made herein are based on our current expectations and we undertake no duty or obligation to update or revise any forward-looking statements for any reason, except as required by law.
VBI Contact
Nicole Anderson, Communications Executive
Phone: (617) 830-3031 x124
Email: info@vbivaccines.com
VBI Investor Contact
Nell Beattie
Chief Business Officer
Email: IR@vbivaccines.com
VBI Media Contact
Burns McClellan, Inc.
Robert Flamm, Ph.D.
Phone: (212) 213-0006
Email: rflamm@burnsmc.com