VBI Vaccines Inc. (Nasdaq: VBIV) (VBI), a commercial-stage biopharmaceutical company developing next-generation infectious disease and immuno-oncology vaccines, today announced the presentation of two abstracts featuring data from the Phase 3 program evaluating Sci-B-Vac®, the company’s tri-antigenic prophylactic hepatitis B (HBV) vaccine, in a late-breaker oral presentation and a poster presentation at The Digital International Liver CongressTM 2020 (ILC), the Annual Meeting of the European Association for the Study of the Liver (EASL), which took place on August 27-29, 2020.
Late-Breaker Oral Presentation
Title: High HBsAb titers consistent across 3 lots of the tri-antigenic HepB Vaccine, Sci-B-Vac®: Results from the second pivotal Phase 3 double-blind, randomized controlled trial designed to assess the lot-to-lot consistency of Sci-B-Vac® in adults (CONSTANT)
During the presentation, Adam Finn, M.D., Ph.D., Professor of Paediatrics at the University of Bristol, UK, and principal investigator of the CONSTANT Phase 3 clinical study, discussed the successfully-met primary endpoint of CONSTANT – demonstration of consistency of immune response as measured by the geometric mean concentration (GMC) of hepatitis B antibodies (anti-HBs titers) across three consecutively-manufactured lots of vaccine. Additional data highlighted included:
- High anti-HBs titers: GMC of anti-HBs for Sci-B-Vac was more than 7.5x compared to Engerix-B after 2 vaccinations (day 168) and more than 3x after 3 vaccinations (day 196)
- Rapid Onset of Seroprotection: After 2 vaccinations, Sci-B-Vac elicited a 90.4% seroprotection rate (SPR) compared to 51.6% with Engerix-B, increasing to 99.3% vs. 94.8% after the 3rd dose
- Safety: No new or unexpected safety signals seen with either study vaccine
“Currently-licensed hepatitis B vaccines, while effective, are not optimal. There is a need to improve their immunogenicity, reliability, and protection rates. Looking back at the data from the CONSTANT study, it’s clear that the immunogenicity of the tri-antigenic vaccine, Sci-B-Vac®, is significantly higher than that of the mono-antigenic vaccine, Engerix-B®,” said Dr. Finn. “More than 90% of individuals vaccinated with Sci-B-Vac® in the CONSTANT study achieved seroprotection after the 2nd dose compared to roughly 50% with the currently licensed vaccine, Engerix-B®.”
A recording of the presentation and copy of the slides are available on the “Events/Presentations” page in the “Investors” section of VBI’s website.
Poster Presentation
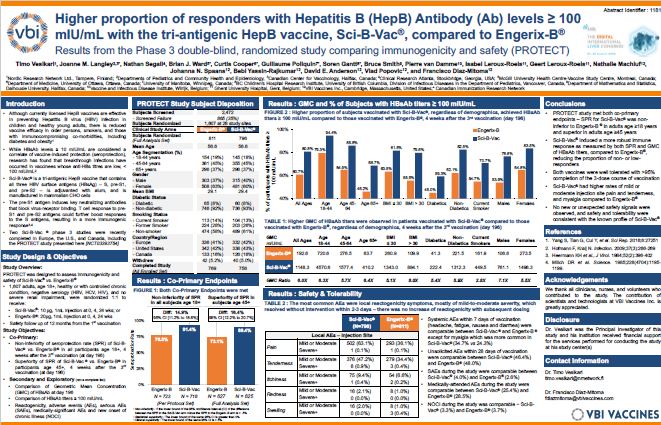
Title: Higher proportion of responders with Hepatitis B Antibody levels ≥ 100 mIU/mL with the tri-antigenic HepB vaccine, Sci-B-Vac®, compared to Engerix-B®: Results from the phase 3 double-blind, randomized study comparing immunogenicity and safety (PROTECT)
Timo Vesikari, M.D., Ph.D., Professor Emeritus and Director of the Nordic Vaccine Research Network in Finland, and principal investigator of the PROTECT and CONSTANT Phase 3 clinical studies, delivered the poster presentation. During the discussions, Dr. Vesikari highlighted Sci-B-Vac®’s ability to induce a more immunogenic response, defined as anti-HBs titers ≥ 100 mIU/mL, across a greater proportion of subjects compared to Engerix-B® in the PROTECT study. The data included:
- Sci-B-Vac induced anti-HBs titers ≥ 100 mIU/mL in more subjects compared to Engerix-B (80.8% vs. 60.7%), with sustained improvement across demographic subgroups including age, BMI, diabetic status, smoking status, and gender.
- Sci-B-Vac elicited 6x higher anti-HBs titers in all subjects compared to Engerix-B (1148.3 mIU/mL vs. 192.6 mIU/mL), again with sustained improvement across age, BMI, diabetic status, smoking status, and gender subgroups.
“The PROTECT study included an analysis of the more stringent anti-HBs titer threshold of 100 mIU/mL – in addition to the standard 10 mIU/mL threshold typically considered the correlate of seroprotection – to examine serological responses across the whole study population as well as various sub-populations,” said Dr. Vesikari. “In the elderly and those with co-morbidities, such as diabetes and obesity, the conventional, licensed hepatitis B vaccine, Energix-B®, induced inadequate immune responses. The responses to the tri-antigenic vaccine, Sci-B-Vac®, were more robust as measured both by seroconversion rate and by anti-HBs titers. This data implies that Sci-B-Vac may be an improved alternative for adult vaccination against Hepatitis B.”
A copy of the e-poster is available on the “Events/Presentations” page in the “Investors” section of VBI’s website.
About VBI Vaccines Inc.
VBI Vaccines Inc. (Nasdaq: VBIV) is a commercial-stage biopharmaceutical company developing a next generation of vaccines to address unmet needs in infectious disease and immuno-oncology. VBI is advancing the prevention and treatment of hepatitis B, with: (1) the only tri-antigenic hepatitis B vaccine, Sci-B-Vac®, which is approved for use and commercially available in Israel and recently completed its Phase 3 program in the U.S., Europe, and Canada; and (2) an immunotherapeutic in development for a functional cure for chronic hepatitis B. VBI’s enveloped virus-like particle (eVLP) platform technology enables development of eVLPs that closely mimic the target virus to elicit a potent immune response. VBI’s lead eVLP programs include a vaccine immunotherapeutic candidate targeting glioblastoma (GBM), a prophylactic cytomegalovirus (CMV) vaccine candidate, and a prophylactic pan-coronavirus vaccine candidate. VBI is headquartered in Cambridge, MA, with research operations in Ottawa, Canada, and research and manufacturing facilities in Rehovot, Israel.
Cautionary Statement on Forward-looking Information
Certain statements in this press release that are forward-looking and not statements of historical fact are forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are forward-looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). The Company cautions that such statements involve risks and uncertainties that may materially affect the Company’s results of operations. Such forward-looking statements are based on the beliefs of management as well as assumptions made by and information currently available to management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, including but not limited to, the impact of general economic, industry or political conditions in the United States or internationally; the impact of the ongoing COVID-19 pandemic on our clinical studies, manufacturing, business plan, and the global economy; the ability to establish that potential products are efficacious or safe in preclinical or clinical trials; the ability to establish or maintain collaborations on the development of therapeutic candidates; the ability to obtain appropriate or necessary governmental approvals to market potential products; the ability to obtain future funding for developmental products and working capital and to obtain such funding on commercially reasonable terms; the Company’s ability to manufacture product candidates on a commercial scale or in collaborations with third parties; changes in the size and nature of competitors; the ability to retain key executives and scientists; and the ability to secure and enforce legal rights related to the Company’s products. A discussion of these and other factors, including risks and uncertainties with respect to the Company, is set forth in the Company’s filings with the SEC and the Canadian securities authorities, including its Annual Report on Form 10-K filed with the SEC on March 5, 2020, and filed with the Canadian security authorities at sedar.com on March 5, 2020, as may be supplemented or amended by the Company’s Quarterly Reports on Form 10-Q. Given these risks, uncertainties and factors, you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. All such forward-looking statements made herein are based on our current expectations and we undertake no duty or obligation to update or revise any forward-looking statements for any reason, except as required by law.
VBI Contact
Nicole Anderson
Director, Corporate Communications & IR
Phone: (617) 830-3031 x124
Email: IR@vbivaccines.com